Pipeline
Topical Roflumilast Foam
(ARQ-154)
Clinical development
Topical roflumilast foam (ARQ-154) is a foam formulation of a highly potent and selective PDE4 inhibitor (roflumilast) which Arcutis is developing for the treatment of inflammatory dermatoses, particularly in hair-bearing areas of the body, such as the scalp.
PDE4 is an intracellular enzyme that increases the production of proinflammatory mediators and decreases the production of anti-inflammatory mediators and has been implicated in a wide range of inflammatory diseases, including psoriasis, atopic dermatitis, and chronic obstructive pulmonary disease. PDE4 is an established target in dermatology, and other PDE4 inhibitors have been approved by the FDA for the topical and systemic treatment of plaque psoriasis.

Read about one of our FDA approvals.
Scalp and Body Psoriasis
Arcutis believes that roflumilast foam has significant potential as a treatment for scalp and body psoriasis.
Plaque psoriasis impacts 9 million people in the United States, and approximately 40% of individuals have involvement of the scalp.
Arcutis conducted ARRECTOR (A Randomized tRial Employing topiCal roflumilasT foam to treat scalp psORiasis), a parallel-group, double-blind, vehicle-controlled pivotal Phase 3 study to assess the efficacy and safety of roflumilast foam 0.3% or a matching vehicle administered once daily in subjects, aged 12 or older, with scalp and body psoriasis.
In this pivotal Phase 3 study, roflumilast foam significantly improved both scalp and body psoriasis. At week 8, 67.3% of individuals treated with roflumilast foam achieved Scalp-Investigator Global Assessment (S-IGA) success compared to 28.1% of individuals treated with vehicle.
Additionally, 46.5% of individuals treated with roflumilast foam achieved Body-Investigator Global Assessment (B-IGA) success at week 8 compared to 20.8% of individuals treated with vehicle. Once-daily roflumilast foam also demonstrated a favorable safety and tolerability profile. Based on this positive data, Arcutis intends to submit a supplemental NDA to the FDA.
Arcutis’ pipeline will change as molecules move through the drug development process. The safety and efficacy of the investigational agents or investigational uses of marketed products have not been established. These uses have not been approved by the US Food and Drug Administration or other regulatory authorities.
There is no guarantee that these therapies or uses will be commercialized. Some of the content on this page is not intended for users outside the US.

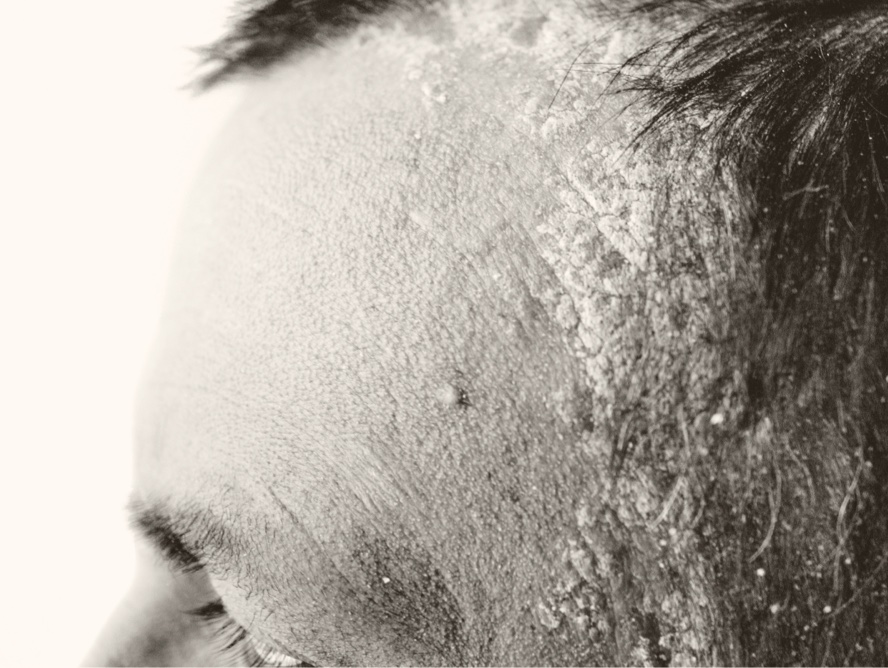
Scalp Psoriasis
Additionally, 46.5% of individuals treated with roflumilast foam achieved Body-Investigator Global Assessment (B-IGA) success at week 8 compared to 20.8% of individuals treated with vehicle. Once-daily roflumilast foam also demonstrated a favorable safety and tolerability profile. Based on this positive data, Arcutis intends to submit a supplemental NDA to the FDA.
Clinical Trials
Completed
Meaningful innovation
at your fingertips.
Read scientific publications.
View